Lucia Fontana1, Juan J. Cirio2, Pedro Lylyk3, Gaston A. Rodriguez-Granillo1;*
1Department of Cardiovascular Imaging, Instituto Medico ENERI, Clínica La Sagrada Familia, Buenos Aires, Argentina
2Stroke Unit, Instituto Medico ENERI, Clínica La Sagrada Familia, Buenos Aires, Argentina
3Department of Interventional Neuroradiology, Instituto Medico ENERI, Clínica La Sagrada Familia, Buenos Aires, Argentina
*Correspondence: [email protected] (Gaston A. Rodriguez-Granillo)
DOI:10.31083/j.rcm.2021.01.215
This is an open access article under the CC BY 4.0 license https://creativecommons.org/licenses/by/4.0/).
Submitted: 19 January 2021 Revised: 12 March 2021 Accepted: 19 March 2021 Published: 30 March 2021
La estrecha relación entre el corazón y el cerebro ha ganado en atención y hoy es reconocida bajo el concepto de Neuro-cardiología. La injuria miocárdica es común en la enfermedad cerebro-vascular y la complicación cardiovascular (CV) es la segunda causa de mortalidad luego de un ACV. Por su parte, la Tomografía computada (TC) es un método no invasivo confiable para el estudio de fuentes cardioembólicas.
En esta Revisión, podremos analizar la importancia de la TC espectral de doble energía, que
mejora significativamente la caracterización tisular y permite, además, una reducción del
volumen de contraste necesario para cada estudio. En particular, es de gran utilidad para el
estudio de fuentes cardio-embólicas, incluyendo la detección de trombos, así como la
estimación del riesgo CV de pacientes con ACV.
The complex and reciprocal relationship between the brain and the heart has gained increasing attention under the concept of neurocardiology.
Myocardial injury is common in cerebrovascular disease, and cardiovascular complications are the second leading cause of death after stroke. Cardiac computed tomography (CT) is a fast and reliable non-invasive tool for the assessment of cardioembolic sources. Compared to single energy CT, spectral/dual energy cardiac CT improves tissue characterization and also leads to significant reductions in contrast volume. In this review article, we portray the potential clinical applications of spectral CT in neurocardiology, focusing in the enhanced diagnosis of cardioembolic sources and cardiovascular risk assessment of patients with stroke, including improved detection of thrombus, identification of subtle myocardial disease, and pulmonary complications within the same session.
Keywords
Imaging; Dual-energy; Stroke; Embolic; Tako-Tsubo
Introduction
The heart and the brain are deeply interrelated both in health and disease, involving diverse physiological and pathophysiological aspects that can lead to brain injury causing
myocardial damage and viceversa [1]. Such complex relationship has recently gained attention under the concept of neurocardiology. Myocardial injury is common in cerebrovascular disease, irrespective of preceding cardiac conditions.
Indeed, ischemic heart disease is the second leading cause of death in patients with nonfatal (ischemic or hemorrhagic) stroke, and mortality rates related to all cardiovascular
diseases is nearly fourfold higher among these patients compared to the general population [2]. Early investigation of the underlying mechanisms of stroke, and particularly the identification of cardioembolic stroke, is important for proper patient treatment as well as to evaluate the possible cardiovascular complications related to clinical outcome.
Cardiac computed tomography (CT) has gained a prominent role as a frontline strategy in low to intermediate risk patients with suspected coronary artery disease [3–5]. Relevant
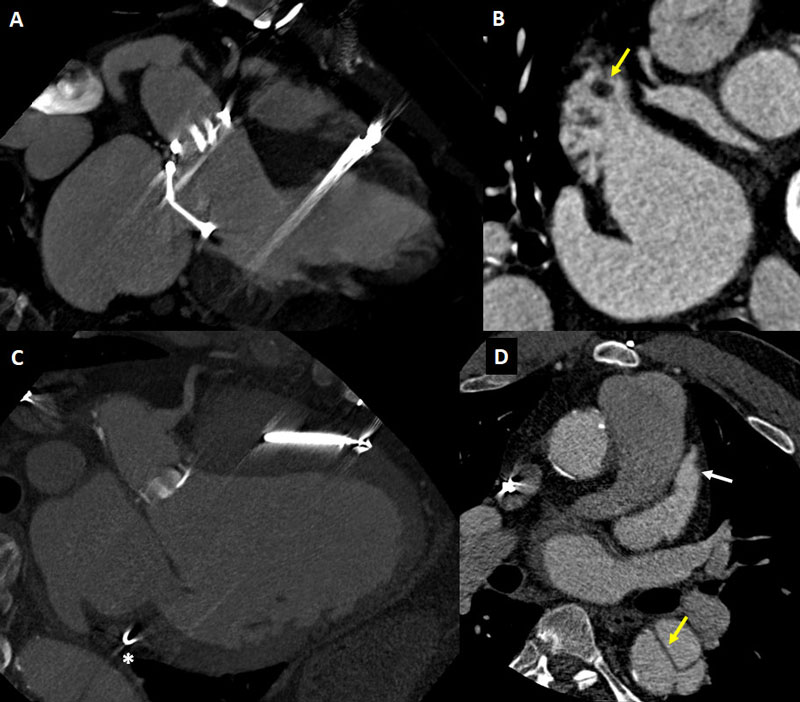
to the field of neurocardiology, cardiac CT provides a reliable, fast, volumetric, non-invasive tool for the assessment of cardioembolic sources (Figs. 1-3). These aspects are critical since most patients with stroke have a number of characteristics including impaired sensory that can lead to gastrointestinal and pulmonary complications, which might potentially be worsened or promoted by transesophageal echocardiogram (TEE) [6, 7].
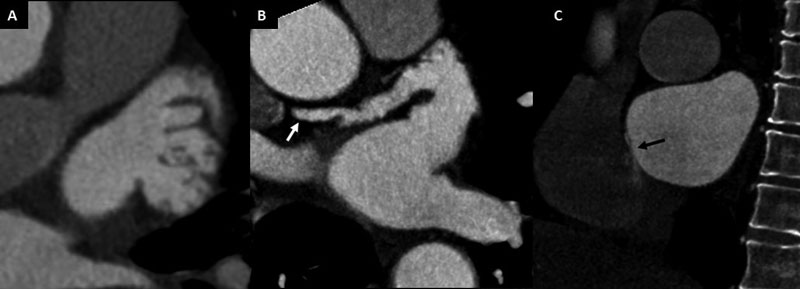
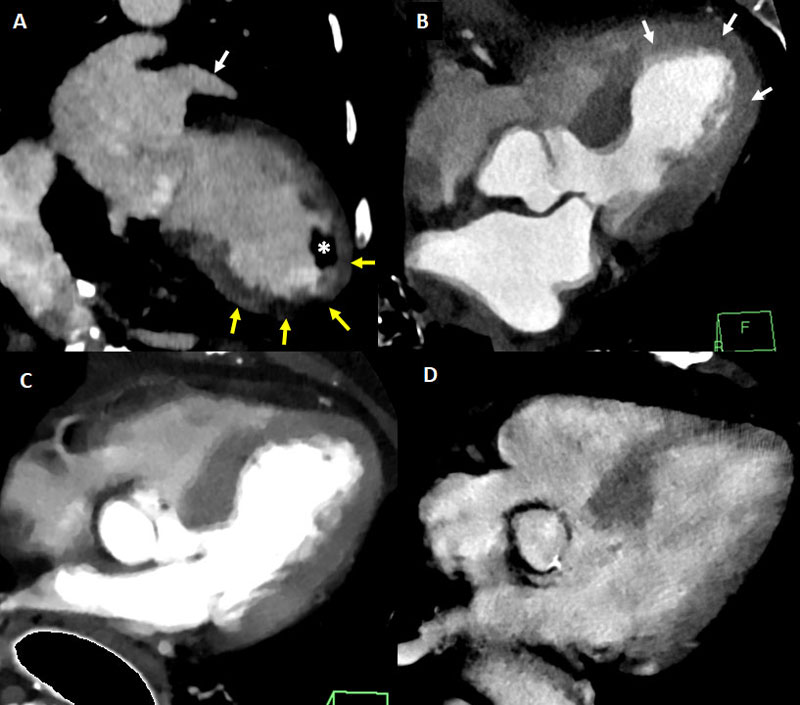
Moreover, a number of cardioembolic sources less amenable for accurate assessment with TEE such as the left ventricular apex, the distal ascending aorta, and subtle myocardial
disease can be evaluated with cardiac CT (Figs. 4, 5).
Despite this, conventional single energy CT has some technical limitations related to the polychromatic nature of X-rays, which lead to beam hardening artifacts that can obstruct precise tissue characterization, particularly mimicking myocardial perfusion defects [8].
Spectral or dual energy cardiac CT has the ability to mitigate these limitations and improve tissue characterization since it enables enhanced intravascular signal density levels,
therefore also leading to significant reductions in contrast volume (Fig. 6) [9–12].
Accordingly, the aim of this review article was to portray the potential clinical applications of spectral CT in neurocardiology, focusing in the diagnosis of cardioembolic sources and cardiovascular risk assessment of patients with stroke.
Technical rationale for spectral cardiac CT in neurocardiology
Tissue characterization using conventional CT is based on single-energy X-ray attenuation levels (Hounsfield units).
Therefore, tissues with completely different chemical composition such as calcium and iodine can be difficult to discriminate.
Spectral or dual energy CT takes advantage of the energy-dependence of X-ray attenuation to achieve an increased contrast attenuation (Fig. 6) and material decomposition,
including quantitative assessment [13–15]. Spectral CT can be obtained using different approaches, briefly broken down into source-based (image acquisition with different tube voltage either by ultrafast kV-switching, dualsource scanner, or by splitting the incident X-ray beam) and detector-based (dual-layer). One of the main differences between these approaches is that detector-based scanners do not require a pre-decision to acquire images in the spectral mode. Regardless of the approach, this technique enables the discrimination between the two mechanisms by which Xrays interact with matter (Compton scatter and photoelectric effect), thereby offering improved tissue characterization.
Dual-layer spectral CT uses two layers (sandwich) of detectors with different photon-absorption capacity; the top layer detecting low-energy photons and the bottom layer detecting high-energy photons. Accordingly, in addition to conventional (polychromatic) images, two distinct energy data sets are provided thus generating multiparametric data that include virtual monoenergetic imaging (VMI), iodine maps, and virtual non-contrast images among other, aimed at diverse clinical applications [16].
VMI at low energy levels (40-55 keV) provides great increase in contrast attenuation levels (Fig. 7), thus allowing increased sensitivity for late iodine detection and major reductions
in iodinated contrast dose [10, 15]. The latter is clinically relevant in the context of neurocardiology imaging since this population usually involves patients with multiple cardiovascular risk factors at risk of contrast-induced nephropathy [17]. Indeed, state-the-art acute ischemic stroke care generally involves CT angiography and if indicated, invasive angiography; leading to an increased likelihood of acute kidney injury [18]. Besides, low VMI can salvage suboptimal vascular studies resulting from technical errors or contrast extravasation.
In parallel, low-energy VMI enables improved discrimination between tissues, being this an advantage among these patients. On one hand, enabling a better identification of thrombus (Figs. 4-9) including improved discrimination between left atrial appendage (LAA) thrombus and stasis (Figs. 8-10), and the detection of subtle myocardial disease (Figs. 7, 11) [19]. And on the other hand, improving carotid plaque characterization and thus potentially enhancing risk stratification of patients with intermediate carotid stenosis;
although this remains to be explored (Fig. 12) [20, 21].
At the other end of the VMI spectrum, mid to high energy levels (> 80 keV) attenuate or cancel artifacts that usually affect single energy CT such as beam hardening, thus further
aiding tissue characterization and avoiding potential misdiagnosis [9, 14]. Moreover, calcium blooming is an important artifact in vascular imaging that causes overestimation
of stenosis and inappropriate classification of lesion severity.
Both VMI at high energy levels and virtual non-contrast images can decrease the blooming effect and therefore achieve a more precise assessment of the lumen [15].
The rationale supporting material decomposition relies on the fact that tissues have different attenuation coefficients that depend on the energy levels of the X-ray beam. As a result, one of the multiparametric features derived from spectral CT allows choosing specific components such as iodine or calcium. In the case of iodine, the generation of iodine density maps enables the quantification of iodine concentration of any specific cardiac structure (Fig. 10) [14]. This approach has numerous applications such as quantitative evaluation of myocardial perfusion, and detection and characterization
of cardiac masses and thrombus. Conversely, calcium suppression can be achieved thereby aiding tissue characterization and discrimination between total and subtotal carotid occlusions [15].
Regarding radiation exposure, it is noteworthy that spectral CT does not imply a significant increase in radiation dose compared to conventional CT. Indeed, dual-layer spectral CT
is acquired using the standard procedure thus using the same radiation dose as in conventional CT. Furthermore, by enabling virtual non-contrast images, the non-contrast phase commonly used for the evaluation of acute aortic syndromes or for the surveillance of endovascular or valve prosthesis among other, might be avoided [22].
Imaging strategies in cardioembolic stroke
Despite the etiology of ischemic stroke is heterogeneous, approximately up to 30% of strokes result from cardiac or aortic origin. This figure* will possibly increase in the upcoming years given the continuing improvements in medical treatment for hypertension and hypercholesterolemia, which are risk factors more related to non-cardioembolic ischemic strokes [23]. The identification of a cardioembolic source is essential for selecting the appropriate therapeutic management, particularly related to the indication and/or strategy of anticoagulation [24]. The main risk factors for cardioembolic
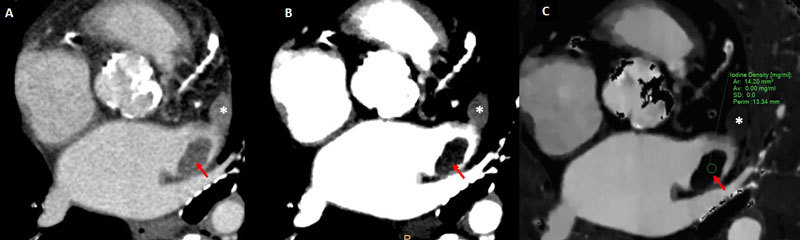
stroke comprise atrial fibrillation (AF), systolic dysfunction, (recent) myocardial infarction, paradoxical emboli through a patent foramen ovale (PFO, Fig. 2), complex aortic arch
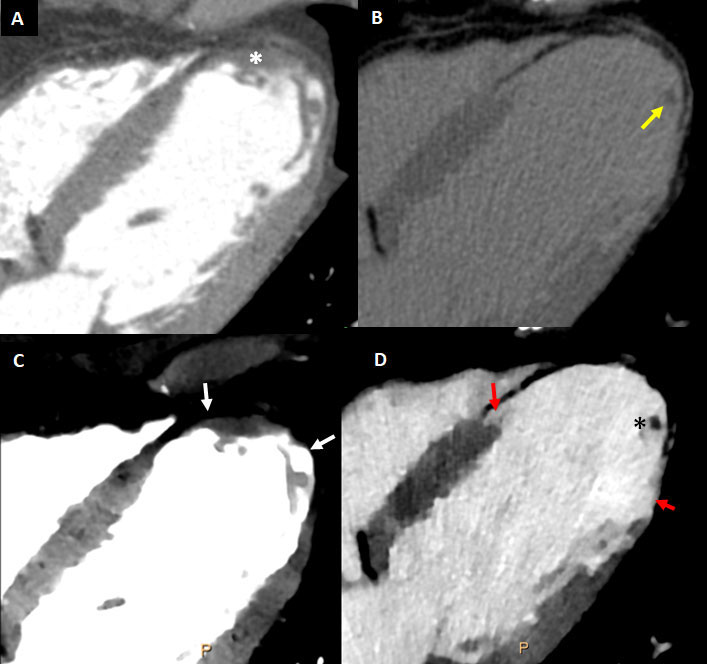
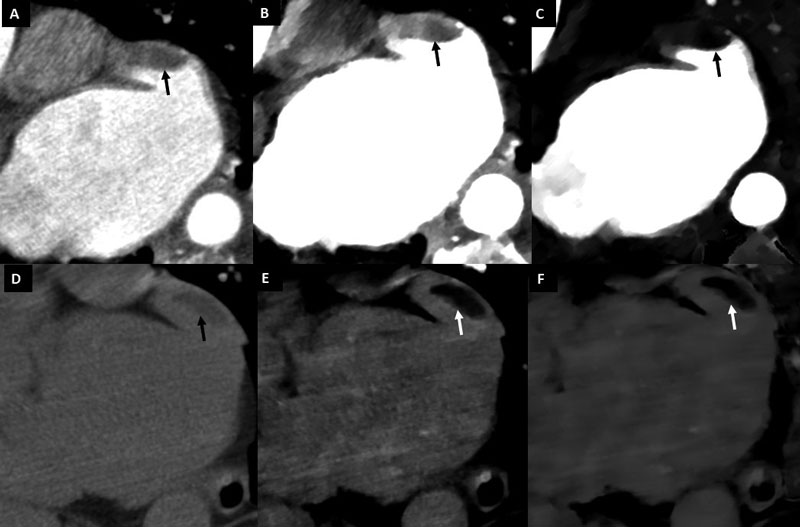
atherosclerotic plaques, mechanical prosthetic heart valves, infective endocarditis and other causes such as cardiac tumors (myxoma and papillary fibroelastoma) and mitral calcification [23]. Among these, LAA thrombus (Figs. 1, 8, 9, 10) secondary to AF or flutter represent the underlying cause in roughly half of all cardioembolic strokes [25].
With regard to specific cardiac imaging in patients with stroke, transthoracic echocardiography (TTE) is the most frequent test for detecting cardiac abnormalities. Despite obvious advantages related to its portability and low cost, TTE is limited for the purpose of ruling out cardioembolic sources. Aside from acoustic window limitations, enhanced among these patients given the restricted possibility to modify the supine position, TTE is a suboptimal technique for the detection of LAA thrombus and aortic plaque.
Conversely, TEE offers improved imaging of LAA and the aorta and is, to date, the reference standard imaging modality for ruling out cardioembolic sources.
Nonetheless, although rare, TEE can been associated to complications including esophageal perforation, infection, oropharygeal and dental trauma, nonfatal arrhythmias, and
adverse reactions related to sedation [7, 26]. The potential risks associated with TEE, and particularly the risk of esophageal lesions, might be incremented among patients
with stroke who can be affected by impaired sensory. In this regard, the recent randomized Transesophageal Echocardiography Dysphagia Risk in Acute Stroke (TEDRAS) trial comprising stroke patients showed a significant increase in
dysphagia after TEE [6].
In parallel, cardiac CT has been positioned in the past decade as a validated and less invasive alternative diagnostic tool for ruling out cardioembolic sources.
Overall, about 40% of acute ischemic strokes are evaluated with either TEE or cardiac CT for this purpose, and in approximately 20-30% of cases a major source of embolism is
detected, being AF or LAA or left ventricular thrombi the most frequent underlying etiology [27, 28].
Cardiac CT for ruling out cardiac thrombi
As aforementioned, TEE is the most widely validated diagnostic tool for the detection of LAA thrombus. Notwithstanding, TEE might offer a deficient or inconclusive assessment in certain specific anatomic scenarios such as multilobed appendages, prominent pectinate muscles, or in case of thrombus at the tip of the LAA (Fig. 2) [29].
Cardiac CT is a less invasive tool that has a good diagnostic capability for detecting intracardiac thrombus and evaluating the aorta. In a meta-analysis including 19 studies and 2,955 patients who underwent TEE and cardiac CT prior to pulmonary vein isolation or cardioversion for AF, or after an ischemic stroke, the sensitivity, specificity, and accuracy for
detection of thrombus by TEE were 96%, 92%, and 99%, respectively, with positive predictive value of 41% and negative predictive value of 94% [30]. Among the subset of patients (n = 753) undergoing delayed-phase acquisition (Fig. 3), the sensitivity, specificity, and accuracy were almost 100%, and the positive predictive value increased to 92%.
The reason for such difference is attributed to the fact that using single-phase (arterial) CT a non-negligible number of cases have incomplete opacification of the LAA, related partly
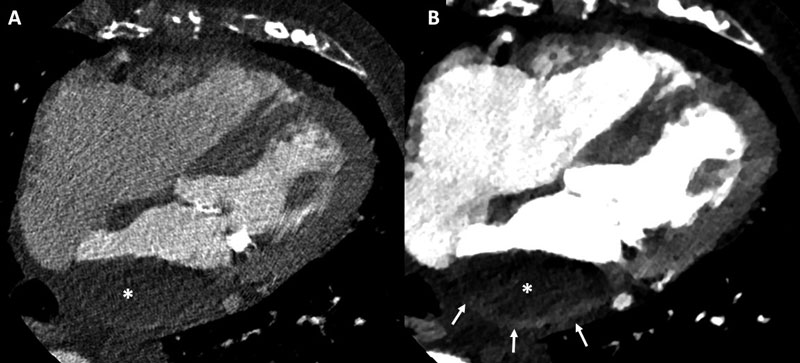
to the supine position of the patient and thereby the anterior position of the LAA (Figs. 3, 8). Also for this reason, cerebrovascular CT angiography extended to include the LAA
might lead to equivocal findings.
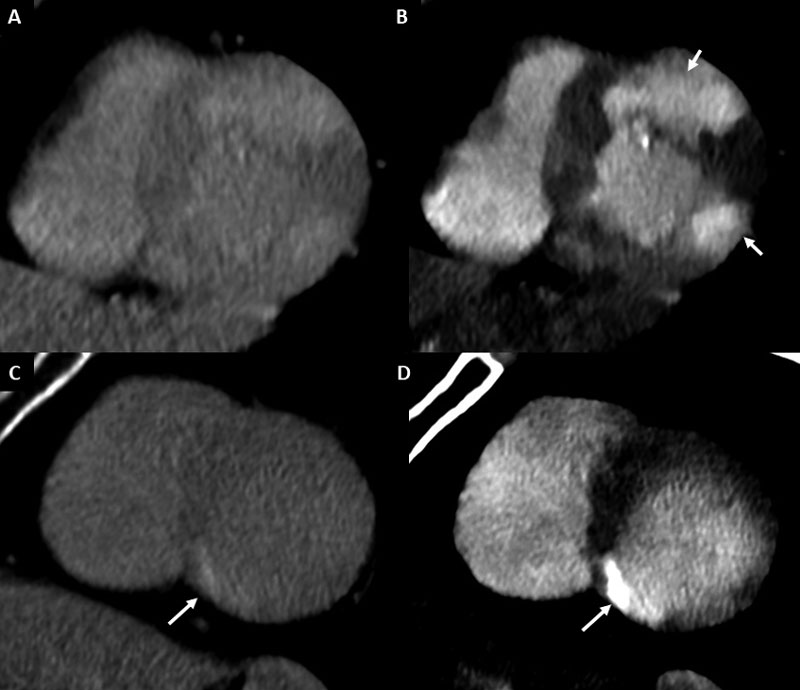
Such filling defects, unless a thrombus is present, resolve with delayed-phase acquisitions (Figs. 3, 8A) and represent slow-flow phenomena/stasis, also referred as spontaneous
echo contrast in TEE. In a recent study including 260 patients with AF referred to pulmonary vein ablation, TEE demonstrated LAA spontaneous echo contrast in 20% of cases and cardiac CT showed an early filling defect in 24% of patients.
Remarkably, LAA thrombus was identified in only 4%, with 100% agreement between techniques. Furthermore, the authors tested different timing of delayed acquisitions at 1-, 3-, and 6-min after contrast administration, and demonstrated the best agreement using images acquired 6 min after contrast [31].
The discrimination between slow-flow from LAA thrombus can be intricate and, as aforementioned, require multiple acquisitions or specific protocols [32]. In this regard, the spectral CT technology has emerged as a means to measure specific iodine concentrations (Fig. 10) and thereby potentially aiding such distinction.
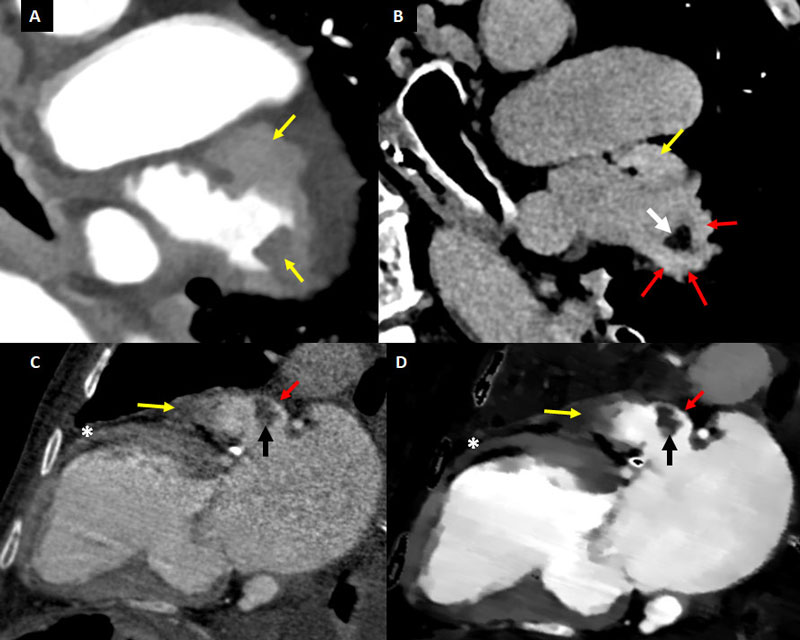
Iodine concentration differs significantly between normal blood flow (i.e. aortic), slow-flow, and thrombus. Spectral CT has the ability to perform specific measurement of local iodine concentrations at any region of interest, both during arterial and delayed-phase images. This enables a clearer discrimination between thrombus and stasis.
In one of the first studies exploring this, Hur et al. demonstrated a mean iodine concentration of 1.23 +- 0.34 mg/mL for thrombus and of 3.61 +- 1.01 mg/mL for spontaneous echo contrast (P = 0.001) [19]. In a more recent study, Li et al. reported an improved diagnostic accuracy using iodine concentration compared to conventional attenuation measurements for detecting LAA thrombus [33]. Nonetheless, the optimal cutoff values for discrimination between blood, slow-flow, and thrombus remain uncertain. In this regard, the LAA-ascending aorta Hounsfield unit ratio threshold for differentiating thrombus from stasis, though variable, is generally below around 0.20 [19].
Late iodine enhancement and left ventricular thrombus
Acute myocardial infarction is a long established risk factor of ischemic stroke, usually related to a left ventricular thrombus overlying areas of wall motion abnormalities.
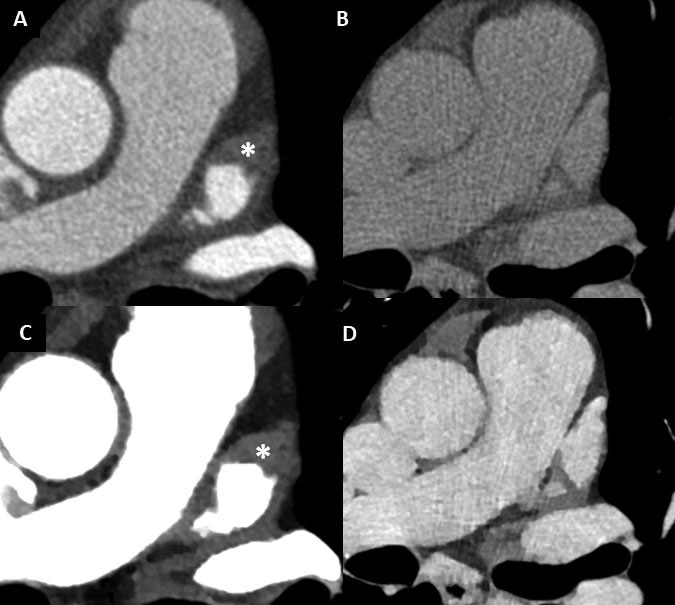
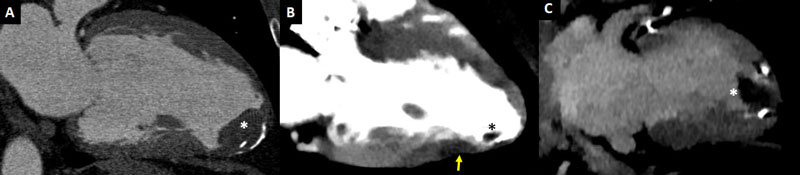
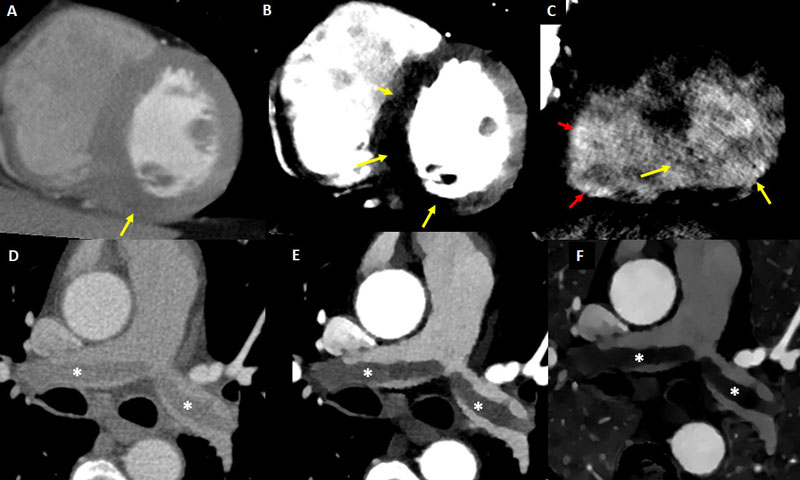
Left ventricular thrombus (Figs. 4, 5) are relatively common among patients with anterior-wall myocardial infarction, with an estimated prevalence of 15-20% [34, 35]. Transthoracic and particularly transesophageal echocardiography have lower sensitivity for the detection of left ventricular thrombus compared to other tools with improved tissue characterization and devoid from acoustic window limitations such as cardiac magnetic resonance (MR) or CT [36, 37]. Singlephase imaging using conventional cardiac CT enables accurate identification of LV thrombus (Fig. 4), and a threshold below 65 Hounsfield units has been reported to convey a sensitivity of 94% and a specificity of 97%. Moreover, as in cardiac MR, late enhancement images provide a much better discrimination
between thrombus and the myocardium (Fig. 5) [38].
In this regard, CT delayed-enhancement (CTDE) imaging shares the same principle than late gadolinium enhancement (LGE) with cardiac MR, with similar contrast kinetics [39]. Therefore, as in LGE, the presence of myocardial late iodine enhancement (LIE) (Figs. 5-7, 11) is a predictor of systolic dysfunction, arrhythmia, major adverse cardiac events, and death; having this been demonstrated in diverse populations including ischemic and non-ischemic cardiomyopathy [40–44]. From a pathophysiological basis, LIE represents an
expansion of the extracellular volume (ECV) associated to irreversible myocardial damage or fibrosis [45].
CTDE can be performed after coronary CT angiography generally using prospective ECG-gating thus involving a low radiation dose, in many cases without additional contrast administration, and has a very high agreement with LGE-MRI (sensitivity and specificity over 90%) [40–42, 46].
Spectral CT offers improved tissue characterization compared to conventional CT, both in terms of myocardial perfusion and delayed-enhancement imaging [41, 42, 46–49].
At first-pass (arterial phase), spectral CT both using iodine density maps and VMI allows more accurate discrimination between normally perfused structures and myocardial perfusion defects (Figs. 4-6, 11, 12). Furthermore, using delayedphase acquisition, particularly 5-7 minutes after contrast administration, improved discrimination between areas of normal myocardium (mildly enhanced, with intermediate iodine concentration), myocardial injury/late iodine enhancement (highly enhanced, similar than intravascular or ventricular cavity), and thrombus (non-enhanced, with minimal or absence of iodine) can be achieved (Figs. 5-7, 11-12).
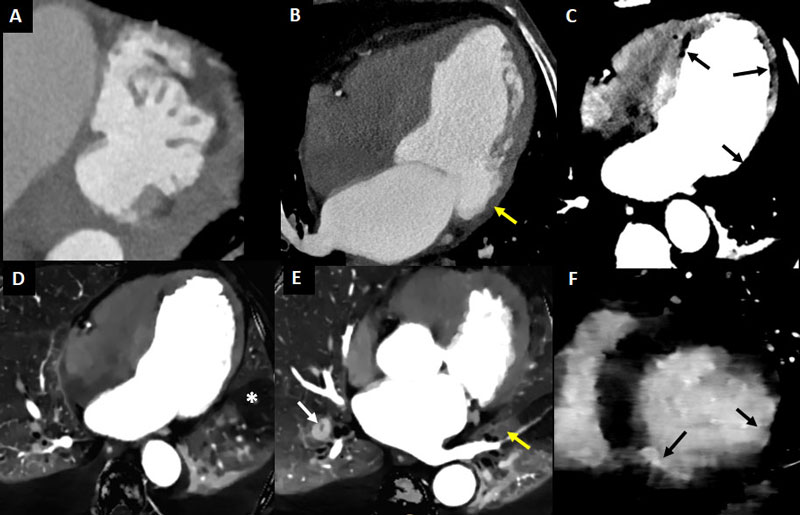
Accordingly, subtle myocardial disease including small infarcts can be detected more easily using spectral compared to conventional CT (Fig. 7).
Aortic thrombi
Almost half of subjects older than 45 years have atherosclerotic plaques in the aorta [50]. Complex (Mayor o igual a 4 mm) aortic atheromatous plaques are a source of acute ischemic stroke, particularly if ulcerated or mobile. However, ulcerated plaques are a relatively common finding in patients with cerebrovascular disease [51]. Aside from the aforementioned limitations of TEE involving the LV apex, the right ventricle, and subtle myocardial infarcts, the thoracic aorta has blinds spots for the assessment with TEE surrounding the distal ascending aorta and the aortic arch. In this regard, Chatzikonstantinou et al. reported a significantly better identification of plaques in the aortic arch using CT angiography [52].
The potential usefulness of spectral over conventional CT for the identification or aortic plaques seems limited to the discrimination between thrombus/plaques and aortic masses,
an exceedingly rare finding [53].
Atrial cardiopathy and pulmonary complications
Atrial cardiopathy is a functional or structural disorder ofthe left atrium that has been associated with LAA stasis or thrombus, even in absence of AF. This condition has an increasingly relevant role in the pathogenesis of embolic stroke of undetermined source (ESUS), a concept that will be addressed in the following section [54]. Atrial cardiopathy may be defined using biomarkers, electrocardiographic indexes and the detection of cardiac fibrosis [54, 55].
LGE using MRI has shown to provide a pre-procedural estimation of the extent of atrial fibrosis and therefore to predict outcomes in patients undergoing AF ablation [56].
Nonetheless, atrial fibrosis imaging is challenging due a number of issues. Among these, the atrial wall is substantially thinner than the ventricular wall, and the spatial resolution
of LGE-MRI is approximately 1.5 mm. Accordingly, spectral CTDE might potentially provide a good alternative for this purpose given a better (sub-millimetric) spatial resolution.
Pulmonary embolism (PE) is a serious complication of stroke (Fig. 11), associated with higher rates of death. A Canadian registry that included 11,287 patients with acute ischemic stroke (AIS), reported a rate of PE of almost 1%, and associated with a higher risk of death at 30 days (26.8% vs. 13.6%; P < 0.001), at 1 year (47.2% vs. 24.6%; P < 0.001), and disability (85.4% vs. 63.6%; P < 0.001) compared to AIS patients without PE [57]. Indeed, subtle signs of PE or deep vein thrombosis are generally overlooked (or misinterpreted as pneumonia or hemiplegic edema for instance) in these patients.
Notably, the first clinical manifestation of PE after stroke in approximately half of the cases is sudden death, underscoring the underestimation and relevance of such condition [58].
Spectral CT using iodine maps offers incremental benefit for the detection of PE (Figs. 6, 11) [59]. Such added value, though modest in patients with suspected PE undergoing CT
pulmonary angiography, might be enhanced in patients with stroke in whom the level of suspicion of PE is lower and its diagnosis can be delayed or even neglected. Moreover, the
increased intravascular enhancement associated with spectral CT (particularly using VMI at low energy levels) enables the detection of PE during cardiac CT not specifically targeted to
evaluate the pulmonary tree (Figs. 6, 11).
Embolic stroke of undetermined source
Approximately one third of strokes have no identifiable etiology after extensive evaluation, and are therefore classified as cryptogenic [60]. Nonetheless, it is currently perceived
that a share of these cryptogenic strokes might actually have undetectable cardioembolic sources, missed by conventional imaging techniques [26].
ESUS represents a large patient group that involves approximately 17% of all ischemic stroke patients. In addition, these patients have a rate of stroke recurrence of 4% to 5% a
year [54]. Diagnosis of ESUS requires exclusion of major cardioembolic sources, as well as proximal occlusive atherosclerosis and lacunar stroke due to cerebral small artery disease [61]. The main pathologies that could be etiologically associated with ESUS are mainly categorized in 7 embolic sources, some of which were previously addressed: 1) atrial cardiopathy, 2) covert atrial fibrillation, 3) left ventricular disease, 4) atherosclerotic plaques, 5) PFO, 6) cardiac valvular disease, and 7) cancer [54].
Cardiac spectral CT might be a useful noninvasive modality for the etiologic assessment of ESUS, particularly for detecting unrecognized or subtle myocardial disease, or embolic
sources that might have been overlooked by other imaging techniques. On top of this, it has been recently suggested that low-dose, non-gated, chest spectral CT performed upon
admission after cerebrovascular CT (without additional contrast administration) might represent an unsophisticated tool for the early triage of cardioembolic sources as well as simultaneously ruling out myocardial disease and pulmonary complications (Figs. 4, 7, 13) [53].
In parallel, spectral CT might be useful for carotid plaque imaging (Fig. 12). Conventional carotid CT angiography has a higher spatial resolution than MRI angiography, lower cost,
is more widely available, and comprises much shorter acquisition time. Notwithstanding, evaluation of diffusely calcified plaques is challenging with conventional CT, overestimating
the degree of stenosis and even leading to inaccurate discrimination between occlusive and sub-occlusive lesions. As aforementioned, spectral CT might overcome some of these
artifacts thereby portraying a more fair assessment of the lumen.
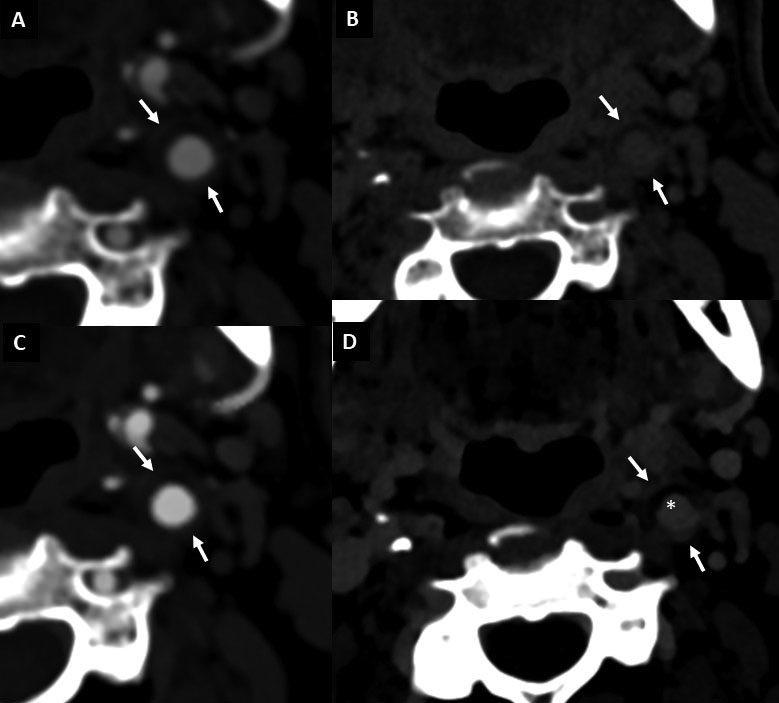
Moreover, spectral CT has shown to improve the discrimination between carotid plaque tissue components, thus potentially aiding risk prediction; albeit this remains to be
established [20, 21]. In this regard, non-significant (< 50% stenosis) carotid lesions have been recognized as an important source of ESUS, with significantly larger non-stenotic carotid plaques ipsilateral compared to contralateral to ischemic stroke (Fig. 12).
Neurogenic stress cardiomyopathy and Tako-Tsubo
The clinical features of cardiac injury in the context of acute brain damage (neurogenic stress cardiomyopathy or neurogenic stunning, NS) and Tako-Tsubo syndrome (TS) are quite similar, although some differences have been described between these conditions. Among other, TS has been reported to involve older patients, a higher rate of ST-segment elevation (with NS more frequently showing T-wave inversion), lower left ventricular ejection fraction, and apical type (while NS showing more frequently a midventricular
or basal type) [62].
Both involve a sudden, transient, and reversible myocardial dysfunction (regional, beyond a coronary territory distribution; very rarely global and in some cases biventricular).
Cardiac enzyme elevation is present in both conditions, predominantly brain natriuretic peptide and mild troponin rise.
Importantly, triggers are clearly different. While TS is caused by psychological stress in the absence of physical damage to the brain, leading to a catecholamine storm, NS results from acute brain injury (generally related to subarachnoid hemorrhage) leading to a catecholamine storm and profound endocrine derangements (Fig. 13) [1, 63].
The autonomic nervous system; acutely affected during brain hemorrhage, has a significant influence on the cardiovascular system due to its ability to modulate heart rate, conduction velocity and cardiac contractility; rapidly increasing systemic vascular resistance and leading to increased left ventricular afterload that can cause, among other, coronary
ischemia. It is therefore not surprising that cardiovascular complications represent the second leading cause of death after stroke [2]. Besides, stroke induced cardiac damage may lead to potentially lifelong cardiac problems related to complications of the forementioned conditions as well as to the incremented likelihood of acute coronary syndromes given the sharing cardiovascular risk factors.
Imaging plays a crucial role in the screening, detection, and characterization of cardiomyopathies. Cardiac CT enables the simultaneous evaluation of coronary arteries, characterization of cardiomyopathy phenotype and quantification of cardiac volumes and function, particularly in patients who are not able to undergo other non-invasive imaging tests such as MRI due to the presence of incompatible pacemakersdefibrillators,
particularly in the acute setting [64].
Spectral CT offers an integral evaluation for TS and NS, improving the characterization of myocardial disease. This technique offers a combination between the features provided
by conventional cardiac CT (coronary tree and functional assessment) and some of those best provided by cardiac MRI (functional assessment and late enhancement) (Fig. 13).
Furthermore, myocardial extracellular volume (ECV) fraction, an emerging surrogate marker of subclinical myocardial disease, has been validated with CT with similar results
compared with cardiac MRI [65, 66]. In this regard, unlike conventional CT, spectral CT allows calculation of the ECV without the need of non-contrast acquisition [45].
Future perspectives
Cardiac CT is a useful modality for detection of embolic sources, comparable to TEE. In addition, it offers additional risk assessment features in patients with stroke within a single
session, such as simultaneous diagnosis of CAD, myocardial perfusion, and LIE; all with incremental prognostic value. Spectral CT might represent a valuable asset in the field of neurocardiology, not only in terms of its ability to reduce iodinated contrast load, but also aided by a significant improvement in tissue characterization. Accordingly, spectral CT shows promise to enable a better thrombus detection and the identification of subtle myocardial disease and/or cardioembolic sources that might be neglected by other imaging
techniques (Fig. 14), thus potentially redefining patients with ESUS.
In parallel, since spectral imaging provides some benefit in terms of improved characterization of carotid plaques (Fig. 12), it might aid risk stratification of intermediate lesions, although the prognostic value of this technique remains uncertain.
Last but not least, the great improvement in tissue characterization provided by spectral imaging combined with the improved temporal resolution of newer generation scanners, might allow the early triage of cardioembolic sources in patients with acute ischemic stroke upon admission immediately after cerebrovascular CT angiography using a low-dose, non-gated, non-contrast chest spectral CT scan (Figs. 4, 7, 13) [53]. Since stroke-related pneumonia is a major cause of increased morbidity and mortality in these patients, using this approach might also be of worth by comprehensively ruling out pulmonary complications within the same session [67].
Overall, spectral CT might enable a more comprehensive cardiovascular evaluation among neurological patients with suspected cardiac injury or cardioembolic sources. Specific
prospective studies are warranted to explore whether this technique might promote an accurate reclassification of stroke etiologies or improve risk assessment, as well as its potential prognostic role in hemorrhagic stroke.
Author contributions
LF conceived and drafted the review article; JJC reviewed the article for critical content; PL reviewed the article for critical content; GRG reviewed the article for critical content.
Ethics approval and consent to participate
All patients or tutors provided informed consent for the anonymous use of their clinical data.
Acknowledgment
We thank two anonymous reviewers for excellent criticism of the article.
Funding
This research received no external funding.
Conflict of interest
None of the authors has conflicts of interest related to this
work.
References
[1] Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brainheart interaction. Circulation Research. 2017; 121: 451-468.
[2] Brønnum-Hansen H, Davidsen M, Thorvaldsen P. Long-term survival and causes of death after stroke. Stroke. 2001; 32: 2131- 2136.
[3] Moss AJ, Williams MC, Newby DE, Nicol ED. The updated NICE guidelines: cardiac CT as the first-line test for coronary artery disease. Current Cardiovascular Imaging Reports. 2017; 10: 15.
[4] Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck- Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. European Heart Journal. 2020; 41: 407-477.
[5] Nissen L, Winther S, Schmidt M, Rønnow Sand NP, Urbonaviciene G, Zelechowski MW, et al. Implementation of coronary computed tomography angiography as nationally recommended first-line test in patients with suspected chronic coronary syndrome:
impact on the use of invasive coronary angiography and revascularization. European Heart Journal – Cardiovascular Imaging. 2020; 21: 1353-1362.
[6] Hamzic S, Braun T, Butz M, Khilan H, Weber S, Yeniguen M, et al. Transesophageal Echocardiography – Dysphagia Risk in Acute Stroke (TEDRAS): a prospective, blind, randomized and controlled clinical trial. European Journal of Neurology. 2021; 28: 172-181.
[7] Hilberath JN, Oakes DA, Shernan SK, Bulwer BE, D’Ambra MN, Eltzschig HK. Safety of transesophageal echocardiography. Journal of the American Society of Echocardiography. 2010; 23: 1115-1111.
[8] Rodríguez-Granillo GA, Rosales MA, Degrossi E, Rodriguez AE. Signal density of left ventricular myocardial segments and impact of beam hardening artifact: implications for myocardial perfusion assessment by multidetector CT coronary angiography. International
Journal of Cardiovascular Imaging. 2010; 26: 345-354.
[9] Rodriguez-Granillo GA, Carrascosa P, Cipriano S, De Zan M, Deviggiano A, Capunay C, et al. Beam hardening artifact reduction using dual energy computed tomography: implications for myocardial perfusion studies. Cardiovascular Diagnosis and Therapy.
2015; 5: 79-85.
[10] Carrascosa P, Capunay C, Rodriguez-Granillo GA, Deviggiano A, Vallejos J, Leipsic JA. Substantial iodine volume load reduction in CT angiography with dual-energy imaging: insights from a pilot randomized study. International Journal of Cardiovascular Imaging.
2014; 30: 1613-1620.
[11] Carrascosa P, Leipsic JA, Capunay C, Deviggiano A, Vallejos J, Goldsmit A, et al. Monochromatic image reconstruction by dual energy imaging allows half iodine load computed tomography coronary angiography. European Journal of Radiology. 2015; 84:
1915-1920.
[12] Shuman WP, Chan KT, Busey JM, Mitsumori LM, Koprowicz KM. Dual-energy CT aortography with 50% reduced iodine dose versus single-energy CT aortography with standard iodine dose. Academic Radiology. 2016; 23: 611-618.
[13] Foley WD, Shuman WP, Siegel MJ, Sahani DV, Boll DT, Bolus DN, et al. White paper of the society of computed body tomography and magnetic resonance on dual-energy CT, Part 2: radiation dose and iodine sensitivity. Journal of Computer Assisted Tomography.
2016; 40: 846-850.
[14] Siegel MJ, Kaza RK, Bolus DN, Boll DT, Rofsky NM, De Cecco CN, et al. White paper of the society of computed body tomograph and magnetic resonance on dual-energy CT, Part 1: technology and terminology. Journal of Computer Assisted Tomography. 2016; 40: 841-845.
[15] De Cecco CN, Schoepf UJ, Steinbach L, Boll DT, Foley WD, Kaza RK, et al. White paper of the society of computed body tomography and magnetic resonance on dual-energy CT, Part 3: vascular, cardiac, pulmonary, and musculoskeletal applications. Journal of Computer Assisted Tomography. 2017; 41: 1-7.
[16] Carrascosa P, Deviggiano A, Rodriguez-Granillo G. Dual energy cardiac CT. Minerva Cardioangiologica. 2017; 65: 265-277.
[17] Rowe AS, Hawkins B, Hamilton LA, Ferrell A, Henry J, Wiseman BF, et al. Contrast-induced nephropathy in ischemic stroke patients undergoing computed tomography angiography: CINISter study. Journal of Stroke and Cerebrovascular Diseases. 2019;
28: 649-654.
[18] Myung JW, Kim JH, Cho J, Park I, Kim HY, Beom JH. Contrastinduced acute kidney injury in radiologic management of acute ischemic stroke in the emergency setting. American Journal of Neuroradiology. 2020; 41: 632-636.
[19] Hur J, Kim YJ, Lee H, Nam JE, Hong YJ, Kim HY, et al. Cardioembolic stroke: dual-energy cardiac CT for differentiation of left atrial appendage thrombus and circulatory stasis. Radiology. 2012; 263: 688-695.
[20] Shinohara Y, Sakamoto M, Kuya K, Kishimoto J, Yamashita E, Fujii S, et al. Carotid plaque evaluation using gemstone spectral imaging: comparison with magnetic resonance angiography. Journal of Stroke and Cerebrovascular Diseases. 2017; 26: 1535-1540.
[21] Reynoso E, Rodriguez-Granillo GA, Capunay C, Deviggiano A, Meli F, Carrascosa P. Spectral signal density of carotid plaque using dual-energy computed tomography. Journal of Neuroimaging. 2017; 27: 511-516.
[22] Danad I, Fayad ZA, Willemink MJ, Min JK. New applications of cardiac computed tomography: dual-energy, spectral, and molecular CT imaging. JACC: Cardiovascular Imaging. 2015; 8: 710-723.
[23] Kamel H, Healey JS. Cardioembolic stroke. Circulation Research. 2017; 120: 514-526.
[24] Fukuda S, Watanabe H, Shimada K, Aikawa M, Kono Y, Jissho S, et al. Left atrial thrombus and prognosis after anticoagulation therapy in patients with atrial fibrillation. Journal of Cardiology. 2011; 58: 266-277.
[25] Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on
Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation): developed in collaboration with the European Heart
Rhythm Association and the Heart Rhythm Society. Circulation. 2006; 114: e257-e354.
[26] Pagán RJ, Parikh PP, Mergo PJ, Gerber TC, Mankad R, Freeman WD, et al. Emerging role of cardiovascular CT and MRI in the evaluation of stroke. American Journal of Roentgenology. 2015; 204: 269-280.
[27] Chung J, Park SH, Kim N, Kim W, Park JH, Ko Y, et al. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification and vascular territory of ischemic stroke lesions diagnosed by diffusion-weighted imaging. Journal of the American Heart Association. 2014; 3: e001119.
[28] Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source: a systematic review and clinical update. Stroke. 2017; 48: 867-872.
[29] Hur J, Kim YJ, Lee H, Nam JE, Ha J, Heo JH, et al. Dual-enhanced cardiac CT for detection of left atrial appendage thrombus in patients with stroke: a prospective comparison study with transesophageal echocardiography. Stroke. 2011; 42: 2471-2477.
[30] Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circulation: Cardiovascular Imaging. 2013; 6: 185-194.
[31] Spagnolo P, Giglio M, Di Marco D, Cannaò PM, Agricola E, Della Bella PE, et al. Diagnosis of left atrial appendage thrombus in patient with atrial fibrillation: delayed contrast-enhanced cardiac CT. European Radiology. 2020; 31: 1236-1244.
[32] Teunissen C, Habets J, Velthuis BK, Cramer MJ, Loh P. Doublecontrast, single-phase computed tomography angiography for ruling out left atrial appendage thrombus prior to atrial fibrillation ablation. International Journal of Cardiovascular Imaging. 2017; 33: 121-128.
[33] Li W, Yu F, Zhu W, Zhang W, Jiang T. Detection of left atrial appendage thrombi by third-generation dual-source dual-energy CT: Iodine concentration versus conventional enhancement measurements. International Journal of Cardiology. 2019; 292: 265- 270.
[34] Solheim S, Seljeflot I, Lunde K, Bjørnerheim R, Aakhus S, Forfang K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. American
Journal of Cardiology. 2010; 106: 1197-1200.
[35] Rodríguez-Granillo GA, Rosales MA, Renes P, Diez E, Pereyra J, Gomez E, et al. Chronic myocardial infarction detection and characterization during coronary artery calcium scoring acquisitions. Journal of Cardiovascular Computed Tomography. 2010; 4: 99- 107.
[36] Weinsaft JW, Kim HW, Crowley AL, Klem I, Shenoy C, Van Assche L, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC: Cardiovascular Imaging. 2011; 4: 702-712.
[37] Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrastenhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. American Heart Journal. 2006; 152: 75-84.
[38] Weinsaft JW, Kim HW, Shah DJ, Klem I, Crowley AL, Brosnan R, et al. Detection of left ventricular thrombus by delayedenhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. Journal of the American
College of Cardiology. 2008; 52: 148-157.
[39] Gerber BL, Belge B, Legros GJ, Lim P, Poncelet A, Pasquet A, et al. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrastenhanced magnetic resonance. Circulation. 2006; 113: 823-833.
[40] Rodriguez-Granillo GA. Delayed enhancement cardiac computed tomography for the assessment of myocardial infarction: from bench to bedside. Cardiovascular Diagnosis and Therapy. 2017; 7: 159-170.
[41] Ohta Y, Kitao S, Yunaga H, Fujii S, Mukai N, Yamamoto K, et al. Myocardial delayed enhancement CT for the evaluation of heart failure: comparison to MRI. Radiology. 2018; 288: 682-691.
[42] Chang S, Han K, Youn J, Im DJ, Kim JY, Suh YJ, et al. Utility of dual-energy CT-based monochromatic imaging in the assessment of myocardial delayed enhancement in patients with cardiomyopathy. Radiology. 2018; 287: 442-451.
[43] Nakamura S, Kitagawa K, Goto Y, Takafuji M, Nakamori S, Kurita T, et al. Prognostic value of stress dynamic computed tomography perfusion with computed tomography delayed enhancement. JACC: Cardiovascular Imaging. 2020; 13: 1721-1734.
[44] Rodriguez-Granillo GA, Rosales MA, Baum S, Rennes P, Rodriguez-Pagani C, Curotto V, et al. Early assessment of myocardial viability by the use of delayed enhancement computed tomography after primary percutaneous coronary intervention. JACC: Cardiovascular Imaging. 2009; 2: 1072-1081.
[45] Lee H, Im DJ, Youn J, Chang S, Suh YJ, Hong YJ, et al. Myocardial extracellular volume fraction with dual-energy equilibrium contrast-enhanced cardiac CT in nonischemic cardiomyopathy: a prospective comparison with cardiac MR imaging. Radiology. 2016; 280: 49-57.
[46] Rodriguez-Granillo GA, Campisi R, Deviggiano A, de Munain MNL, Zan MD, Capunay C, et al. Detection of myocardial infarction using delayed enhancement dual-energy CT in stable patients. American Journal of Roentgenology. 2017; 209: 1023-1032.
[47] Carrascosa PM, Deviggiano A, Capunay C, Campisi R, López de Munain M, Vallejos J, et al. Incremental value of myocardial perfusion over coronary angiography by spectral computed tomography in patients with intermediate to high likelihood of coronary artery disease. European Journal of Radiology. 2015; 84: 637-642.
[48] Carrascosa P, Deviggiano A, de Zan M, Capunay C, Campisi R, Rodriguez-Granillo GA. Improved discrimination of myocardial perfusion defects at low energy levels using virtual monochromatic imaging. Journal of Computer Assisted Tomography. 2017; 41: 661-667.
[49] Arnoldi E, Lee YS, Ruzsics B, Weininger M, Spears JR, Rowley CP, et al. CT detection of myocardial blood volume deficits: dualenergy CT compared with single-energy CT spectra. Journal of Cardiovascular Computed Tomography. 2011; 5: 421-429.
[50] Meissner I, Khandheria BK, Sheps SG, Schwartz GL, Wiebers DO, Whisnant JP, et al. Atherosclerosis of the aorta: risk factor, risk marker, or innocent bystander? A prospective population-based transesophageal echocardiography study. Journal of the American
College of Cardiology. 2004; 44: 1018-1024.
[51] Amarenco P, Duyckaerts C, Tzourio C, Hénin D, Bousser MG, Hauw JJ. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. New England Journal of Medicine. 1992; 326: 221-225.
[52] Chatzikonstantinou A, Krissak R, Flüchter S, Artemis D, Schaefer
A, Schoenberg SO, et al. CT angiography of the aorta is superior
to transesophageal echocardiography for determining stroke subtypes
in patients with cryptogenic ischemic stroke. Cerebrovascular
Diseases. 2012; 33: 322-328.
[53] Rodriguez-Granillo GA, Cirio JJ, Ciardi C, Caballero ML, Diluca
P, Castrillon R, et al. Cardiovascular thrombotic complications
in acute ischemic stroke assessed by chest spectral computed tomography
during COVID-19. Minerva Cardiology and Angiology.
[54] Ntaios G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. Journal of the American College of Cardiology. 2020; 75: 333-340.
[55] Yaghi S, Kamel H, Elkind MSV. Atrial cardiopathy: a mechanism of cryptogenic stroke. Expert Review of Cardiovascular Therapy. 2017; 15: 591-599.
[56] Siebermair J, Kholmovski EG, Marrouche N. Assessment of left atrial fibrosis by late gadolinium enhancement magnetic resonance imaging: methodology and clinical implications. JACC: Clinical Electrophysiology. 2017; 3: 791-802.
[57] Pongmoragot J, Rabinstein AA, Nilanont Y, Swartz RH, Zhou L, Saposnik G. Pulmonary embolism in ischemic stroke: clinical presentation, risk factors, and outcome. Journal of the American Heart Association. 2013; 2: e000372.
[58] Wijdicks EF, Scott JP. Pulmonary embolism associated with acute stroke. Mayo Clinic Proceedings. 1997; 72: 297-300.
[59] Weidman EK, Plodkowski AJ, Halpenny DF, Hayes SA, Perez-Johnston R, Zheng J, et al. Dual-energy CT angiography for detection of pulmonary emboli: incremental benefit of iodine maps. Radiology. 2018; 289: 546-553.
[60] Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American
Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006; 37: 577-617.
[61] Hart RG, Diener H, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurology. 2014; 13: 429-438.
[62] Ancona F, Bertoldi LF, Ruggieri F, Cerri M, Magnoni M, Beretta L, et al. Takotsubo cardiomyopathy and neurogenic stunned myocardium: similar albeit different. European Heart Journal. 2016; 37: 2830-2832.
[63] Tavazzi G, Zanierato M, Via G, Iotti GA, Procaccio F. Are neurogenic stress cardiomyopathy and takotsubo different syndromes with common pathways?: Etiopathological insights on dysfunctional hearts. JACC: Heart Failure. 2017; 5: 940-942.
[64] Kalisz K, Rajiah P. Computed tomography of cardiomyopathies. Cardiovascular Diagnosis and Therapy. 2017; 7: 539-556.
[65] Treibel TA, Bandula S, Fontana M, White SK, Gilbertson JA, Herrey AS, et al. Extracellular volume quantification by dynamic equilibrium cardiac computed tomography in cardiac amyloidosis. Journal of Cardiovascular Computed Tomography. 2015; 9: 585-592.
[66] Nacif MS, Kawel N, Lee JJ, Chen X, Yao J, Zavodni A, et al. Interstitial myocardial fibrosis assessed as extracellular volume fraction with low-radiation-dose cardiac CT. Radiology. 2012; 264: 876-883.
[67] Hannawi Y, Hannawi B, Rao CPV, Suarez JI, Bershad EM. Stroke associated neumonia: major advances and obstacles. Cerebrovascular Diseases. 2013; 35: 430-443.

